Type II diamonds are chemically the purest among all diamonds. The material of these diamonds is almost all carbon without any trace of nitrogen atoms which are usually present in most diamonds.
203 Carat Type II a Millennium Star Diamond

These diamonds are also alluded to as “Golconda” diamonds. Reference is to the legendary Indian Golconda mines of the bygone era that supplied the majority of diamonds of this class.
Famous Golconda Diamonds

You can further break this class into type IIa and type 2b.
Type IIa Diamonds:
Type IIa stones are all carbon devoid of any Internal impurities. People revere these gems for their beauty & color. D-color white stones of this class are whiter than the quality D-color grade stones of other classes. Connoisseurs even refer to them as “Super D” or “C,” albeit these are not official gradings. Type IIa diamonds, when graded as fancy colored diamonds, display intense Yellow, Brown, Orange, Pink, Purple, and Red colors. The colors are because of plastic deformation of crystal lattice.
The 102.39 carat Maiko Star Canadian, Type IIa, D Colour, Flawless Oval Diamond

Type IIb Diamonds:
In Type IIb gems, a few boron atoms swap places with carbon atoms. Boron induces a gorgeous blue color into the stone. Hope Diamond is a paragon of type IIb diamonds.
Recent years have catalyzed interest and excitement for Type II diamonds. Diamond connoisseurs and collectors are showering their love upon these stunning stones.
Notably, research conducted during the last four years has given us immense knowledge about how these diamonds form. In addition, the ongoing research work has taught us a lot about the processes occurring deep inside the earth’s cauldron.
And here is a collection of natural diamonds.
Natural Diamonds

You can appreciate the profusion of the stones’ sizes, shapes, and colors in the above images. Also, it should make you think about the motley of minerals inside the earth and the concoction they cook up to impart these diverse characteristics to the diamonds.
Classification of Diamonds into Type I & Type II:
You can categorize and classify diamonds in different ways based on their characteristics. But the most recognized classification scheme is the type classification. It is a scientific method of classifying diamonds based on the level and type of their chemical impurities.
Classification of Diamonds into Type I and Type II
Under this system, diamonds are divided down into type I and type II depending on the presence of nitrogen or its absence. Type I diamonds contain nitrogen atoms as their main impurity.
The 14.83 ct Fancy Vivid Purple-Pink, IF, Type IIa Spirit of Rose Diamond

Diamonds are crystals of carbon atoms linked together by strong covalent bonds. At times, atoms of other elements replace a few of the carbon ones because of the sordid ambiance of deep regions of diamond formation. Most often, the replacing atoms are those of nitrogen. Analysts detect this nitrogen with infrared spectroscopy. Further, they classify diamonds containing nitrogen impurities as type I. Ninety-eight to ninety-nine percent of all mined diamonds belong to this category.
On the other hand, we also have very pure diamond crystals. They are virtually all carbon. When nitrogen is absent, we classify them as type II.
Dubbed As The Rarest White Diamond Sotheby’s 102.34 ct, D Flawless, Type IIa

Sub-Classification of Diamonds into Type IIa & Type IIb:
Type IIa:
Further, when Boron is also absent from such type II diamonds, we categorize them as type IIa. Thus, absence of nitrogen as well a Boron makes the diamond type IIa.
Type IIb:
When Boron atoms permeate diamond crystals, we categorize them as type IIb. On account of Boron, these diamonds acquire a very desirable blue color. Thus, absence of nitrogen but presence of Boron makes the diamond type IIb.
All type II diamonds command a very high premium price in the gem market and generate intense interest in gemology. Most of the world’s largest and most celebrated diamonds belong to this category.
Pear Shaped, Type IIb Apollo Blue And Type IIA Artemis Pink Sotheby’s

These diamonds not only command a very high premium price in the gem market but also are of great interest in gemology. Most of the largest and celebrated diamonds of the world belong to this category.
The Gemological Institute of America has analyzed millions of diamonds and reached certain conclusions.
- The majority of large-size D flawless diamonds are type 2a diamonds.
- Inclusion free attributes or low inclusion content make these diamonds flawless.
Most of Our Famous Diamonds are Type IIa or Type IIb:
Majority of large size (from above 5 carats up to 20 carats) D flawless diamonds are type 2a diamonds. Data analysis showed that if the top largest diamonds of these were considered they have a propensity towards being D colored and type 2.
It is fascinating to learn that very large D-color flawless diamonds like the Cullinan are almost always type 2a. The most opulent and legendary diamonds on the planet are type 2a or their sibling type 2b containing traces of boron like the Hope Diamond.
Also, a fairly large proportion of type 2 diamonds are flawless. They have an exceptionally low content of inclusions. They are either completely inclusion-free or have very few inclusions compared to type I diamonds of comparable size.
These diamonds are also significant from the point of view of science. Does mother nature cook these unique stones in an extraordinary kitchen using a secret recipe?
Yet We Understand type II Diamonds From Their Scarce Inclusions:
Much of our knowledge about diamond formation comes from studying inclusions within diamonds. The inclusions are a storehouse of information about the site where diamonds grow.
Use your imagination and visualize a baby diamond growing inside the earth’s mantle. As it swells, it may perhaps sheathe some stuff from its surroundings and include a mineral crumb or two. If such a diamond is transported to the earth’s surface riding on a kimberlite eruption, nature preserves the mineral inside the diamond.
You have a snippet of the region of diamond formation and its environment. It conveys an astonishing amount of information about the place and processes of diamond growth. And we learn that diamonds form in the densest parts of continents at a depth of about 150 to 200 kilometers. They arrive at the surface in deep-seated volcanic eruptions known as kimberlite eruptions.
The eruptions build vertical pipe-like structures that penetrate the surrounding rock. These are known as kimberlite pipes. We discover and commercially mine the diamonds in such kimberlite pipes. Kimberlites are intrusive igneous rocks, and scientists agree that they form deep inside the mantle.
We also learn from studying the diamonds and their inclusions that diamonds form in nature in different ways.
So, where do our large type 2a and type 2b diamonds fit in this model of diamond formation? For two good reasons, the answer remained a mystery for a very long time. Firstly, these diamonds are so scarce and valuable that you don’t have the liberty to access them for research purposes. Secondly, these diamonds rarely display any inclusions. It means that you have nothing to work with.
And yet, in the rarest of rare cases, gemologists have observed black scaly-like inclusions in a few type 2 diamonds.
So How Do Gemologists Get Hold of Such Scarce Inclusions:
Senior gemologists at the Gemmological Institute of America (GIA) made use of an immense resource infrastructure already available at their disposal. Hundreds of thousands of diamonds, including type II, come into the institute for grading. And they borrowed these diamonds for research.
In this manner, the GIA’s gemologists could access many of the rarest diamonds they would otherwise not have come across in their lifetime. Also, with the excellent reputation of the institute came great contacts and connections. Through this network, they could also procure trimmings from much larger diamonds exfoliated while the stones were being cut and shaped. Often, these trimmings are from the flawed and included regions of the diamond.
The analysts have identified the geographic origin of these unique gemstones based on results from this research.
Analysis of Inclusions in Type II Diamonds:
The flake-like inclusions under study were metallic in luster and composition. And these are the most common inclusions in type II diamonds.
Moreover, analysts found that the inclusion was a mixture of iron carbide compound, iron-nickel alloy, and iron sulfide compound. So it had a lot of iron and nickel. Some diamonds also included the garnet-calcium-silicate-perovskite phase.
From earlier research and calculations, scientists know these minerals form only under extreme pressure at 400 km to 750 km deep depths.
Remember, we discussed above that, normally, diamonds form at a depth of 150 to 200 kilometers deep in the earth.
On the other hand, we realize that the type II diamonds have formed at 4 to 5 times that immense depth. As such, they are also rightly called superdeep diamonds.
Let us now revisit Hope Diamond which we know is a type II b diamond. Furthermore, we know that the presence of Boron (which imparts the alluring blue) distinguishes these diamonds.
Type IIb diamonds are indeed extraordinarily rare. And yet they are found in mines in diverse locations around the globe. Therefore, we can infer that the geological conditions necessary for these diamonds’ growth are a recurring phenomenon.
We know that Boron is abundant on the surface of the earth. Further, water bodies on the surface redistribute the Boron all over. So earth’s exterior is heavily concentrated with Boron.
But as we go deeper, boron concentration starts diminishing. And at extreme depths, Boron is virtually absent. So, the presence of Boron in diamonds formed in this boron deficient region piques our curiosity. Where does this Boron come from?
How Does Boron Reach These Supe Depths?
To answer, we first need to analyze the inclusions found in type IIb diamonds. These diamonds display a somewhat different mix of inclusions dominated by the calcium-silicate phase.
The most common inclusion identified in the type IIb diamonds examined was CaSiO3-walstromite, interpreted as an inversion product from CaSiO3-perovskite. Some of these inclusions also contained larnite (Ca2SiO4) and, less frequently, CaSi2O5-titanite, wollastonite, and perovskite (CaTiO3).
The second most common inclusion type was pyroxene, with some inclusions containing jeffbenite (TAPP), spinel, and olivine. Scientists interpret these inclusions as retrogressed bridgmanite (MgSiO3-perovskite).
An essential additional inclusion phase in these diamonds is coesite, interpreted as inverted stishovite. These assemblages suggest derivation from a basic, possibly Ca-rich, host rock paragenesis from the lower mantle.
We don’t need to understand the chemical mumbo-jumbo. Rather, we only need to comprehend that inclusions described above are vital indicators of depth. Things that only form at lower mantle depths of around 700 kilometers.
We should also appreciate that mineral compounds that form under high pressure & temperatures at great depths decompose when they come to the surface and the pressure on them is released. So researchers reverse engineer and deduce the original mineral inclusions from their decomposed versions.
We are coming back to our earlier discussion on Boron. We were discussing that even though the earth’s surface has abundant Boron, the great depths are virtually devoid of it. So, from where does the Boron at great depths come?
To answer this question, we have to understand the dynamics of the basalt rock making up most of the ocean floor. We also have to understand a bit about plate tectonics.
Role of Ocean Crust & Plate Tectonics:
On the floor of the ocean, you have the ocean crust. And this crust sprawling over the oceanic lithosphere is usually a rock type known as basalt. This top layer of crust keeps getting pushed deeper and deeper by the process of plate tectonics known as subduction.
As the crust gets pushed deeper and deeper, its mineral content keeps changing with increasing heat and pressure. Over time, the now-metamorphosed basaltic rock from the ocean floor reaches the lower mantle.
Earth’s Interiors
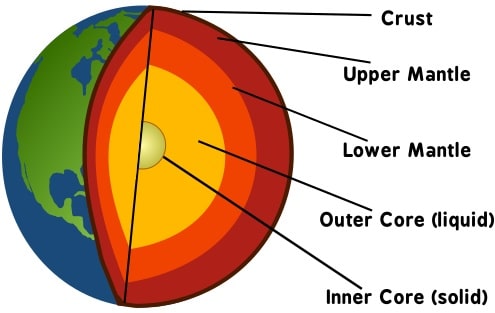
And now for introducing the idea regarding the source of Boron at such inhuman depths. First, you must remember that the presence of Boron marks type IIb diamonds.
Also, let me remind you that the earth’s surface, including the basaltic oceanic tectonic plates, is rich in Boron. And we have seen that these boron-rich plates sink deeper and deeper due to tectonic subduction to the lower mantle and beyond. And this movement is perpetual, like a natural conveyor belt.
Scientists are now theorizing that the oceanic subduction plates transport some quantities of Boron down to lower mantle levels.
Further, when a diamond is seeded and grows in this hotch-potch of chemicals and minerals, it might be impregnated by a few of those boron atoms. Just enough to impart that gorgeous blue color.
We realize that diamonds are also a snapshot of deep earth in addition to gorgeous lovable gemstones. From the metallic inclusions in type II diamonds, we can deduce that deep mantle regions probably hold metallic iron.
We further comprehend that subducting oceanic basaltic plates might carry Boron from the earth’s surface to the deep mantle.
We must also appreciate that diamonds are invaluable sample capsules from the greatest depths of our planet. As such, they are of great consequence for scientific studies.
Read More Articles About Diamonds: